Thiamine, also known as vitamin B1, is an essential nutrient that plays a pivotal role in maintaining our health and well-being. Over the past several years, there has been renewed interest in the possibility of a subclinical efficiency of it as a causative factor in chronic illness in multiple disease states. It can often mimic many other diseases and lead to the misdiagnosis of other chronic conditions such as SIBO and IBS, neuropathy and POTS, neurological disease, chronic fatigue syndrome, and cardiovascular diseases. Therefore I would like to give some background and information on this very important topic so that you may have information you need to make a decision whether or not you want to investigate this further as a potential health issue…
By supporting a myriad of metabolic processes and maintaining the proper functioning of our nervous system, thiamine ensures that our bodies operate at peak efficiency. In this article, we will explore the remarkable benefits of thiamine supplementation and how it can improve your overall health.
Thiamine is crucial for enabling enzymes to function effectively, as it often serves as a vital cofactor. Before it can perform this role, thiamine must be activated through a process called phosphorylation, which produces thiamine pyrophosphate and other phosphate esters.
Once activated, thiamine pyrophosphate contributes to various metabolic processes, including energy production through the Krebs cycle, processing amino acids, and the pentose-phosphate pathway. Thiamine also plays a key role in maintaining the brain and nervous system, where it influences the activity of other enzymes and neurotransmitters.
Thiamine and ATP Synthesis
Thiamine is required for the production of ATP, the primary energy currency of cells. It serves as a cofactor for several enzymes involved in carbohydrate and amino acid metabolism, including pyruvate dehydrogenase and alpha-ketoglutarate dehydrogenase, which play a role in the citric acid cycle. The citric acid cycle generates high-energy electrons that are then used in oxidative phosphorylation (OXPHOS) to synthesize ATP in the mitochondria.
Thiamine and Mitochondrial Energetics: Oxidative Phosphorylation (OXPHOS)
Oxidative phosphorylation (OXPHOS) is a process that takes place within the mitochondria, where high-energy electrons derived from the citric acid cycle are used to generate ATP. Thiamine is essential for the proper functioning of OXPHOS, as it is involved in the generation of NADH and FADH2, which act as electron carriers in the electron transport chain. This chain creates a proton gradient that drives the synthesis of ATP via ATP synthase, thereby providing cells with the energy they require for various biological processes.
Thiamine and Cellular Oxygenation
In addition to its role in energy synthesis, thiamine is also crucial for cellular oxygenation. Mitochondrial oxidation, the process by which cells utilize oxygen to generate ATP, relies on thiamine-dependent enzymes. Insufficient thiamine levels can lead to impaired mitochondrial oxidation, resulting in cellular hypoxia, or low oxygen levels in the cells. This can contribute to a wide range of health problems, from chronic illnesses to acute conditions.
The Consequences of Thiamine Deficiency
A lack of thiamine can have severe consequences on our health. Insufficient levels of this essential nutrient can lead to a reduced rate of cellular energy production and result in diseases such as Beriberi, Wernicke’s Encephalopathy, and Korsakoff’s Syndrome. Factors that contribute to thiamine deficiency include chronic alcohol consumption, a high intake of refined carbohydrates, and the use of certain medications.
Thiamine Deficiency and Dysautonomia
Dysautonomia refers to a malfunction of the autonomic nervous system, which controls involuntary bodily processes such as blood pressure, heart rate, and digestion. Thiamine is particularly important for the proper functioning of the brain regions that coordinate the autonomic nervous system, including the mamillary bodies, thalamus, hypothalamus, brain stem, and cerebellum. Thiamine deficiency can impair energy generation in these regions, leading to dysautonomia and its downstream consequences on various bodily systems.
Thiamine deficiency and neurological conditions
Thiamine deficiency can affect the function of the nervous system and lead to various neurological illnesses, including multiple sclerosis (MS) and Parkinson’s disease. Thiamine is essential for the maintenance of the myelin sheath, which is the protective covering of the nerves. Thiamine deficiency can lead to demyelination, which is the loss of myelin sheath, and nerve damage, leading to neurological symptoms.
Additionally, thiamine deficiency can impair the function of the pyruvate dehydrogenase complex (PDC), which is essential for energy metabolism in the brain. PDC dysfunction can lead to a decrease in energy production, leading to neuronal damage and neurodegeneration.
Antonio Costantini’s work with Parkinson’s and dystonia patients has demonstrated the potential benefits of thiamine supplementation in these neurological conditions. Costantini et al. (2013) conducted a study in which 40 patients with Parkinson’s disease received high-dose thiamine supplementation for three months. The results showed a significant improvement in motor symptoms, cognitive function, and quality of life. In another study, Costantini et al. (2016) administered thiamine supplementation to 14 patients with dystonia and observed a significant improvement in dystonia symptoms.
Several studies have also reported a potential link between thiamine deficiency and MS. Thiamine deficiency can impair the function of the myelin sheath, leading to demyelination, which is a hallmark feature of MS. A study by Lee et al. (2019) reported that thiamine deficiency was prevalent in patients with MS and that thiamine supplementation led to a significant improvement in neurological symptoms.
Thiamine Deficiency and Irritable Bowel Syndrome (IBS) (Including SIBO)
Thiamine is essential for maintaining the health of the gastrointestinal system. IBS, a common gastrointestinal disorder, has been associated with thiamine deficiency. A study found that IBS patients who were given thiamine supplements experienced significant improvement in their symptoms. This suggests that addressing thiamine deficiency may be a potential strategy for managing IBS.
Thiamine’s role in supporting key enzymes responsible for energy production
Transketolase (TKT) and Pyruvate Dehydrogenase Complex (PDC) are two essential enzymes that play significant roles in the proper functioning of the Krebs cycle and fatty acid synthesis. They can be affected by environmental toxins, genetic, and lifestyle factors. Thiamine supplementation can help reverse these issues and support optimal cellular metabolism. In this article, we will discuss the roles of TKT and PDC and the benefits of thiamine supplementation.
Transketolase (TKT)
Transketolase is an enzyme that plays a critical role in the pentose phosphate pathway (PPP). The PPP is a metabolic pathway parallel to glycolysis and is involved in generating ribose-5-phosphate for nucleotide synthesis and NADPH for biosynthesis and cellular redox balance. TKT is responsible for transferring carbon units between sugar molecules and requires thiamine pyrophosphate (TPP) as a cofactor for its activity. . The proper functioning of TKT is crucial for maintaining the balance between energy production and biosynthesis in cells.
Pyruvate Dehydrogenase Complex (PDC)
The Pyruvate Dehydrogenase Complex is a multi-enzyme complex that plays a central role in cellular metabolism, linking glycolysis to the Krebs cycle. PDC is responsible for converting pyruvate, a product of glycolysis, into acetyl-CoA, which enters the Krebs cycle for further metabolism and energy production. This complex also requires TPP as a cofactor, making thiamine an essential nutrient for its activity. PDC is crucial for generating ATP and facilitating fatty acid synthesis.
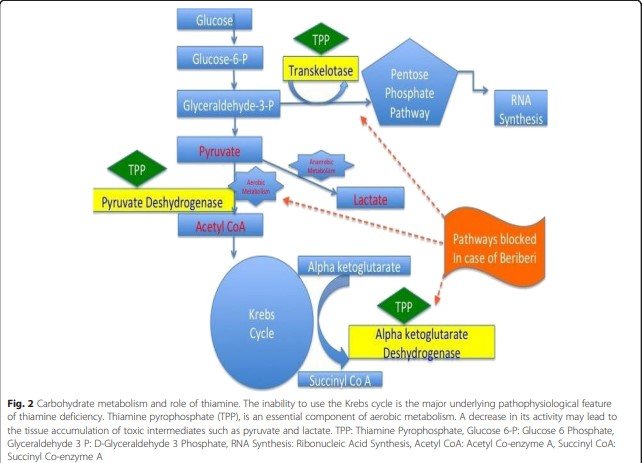
Image from: Dabar, George & Harmouche, Carine & Habr, Bassem & Riachy, Moussa & Jaber, Bertrand. (2015). Shoshin Beriberi in Critically-Ill patients: Case series. Nutrition journal. 14. 51. 10.1186/s12937-015-0039-7.
Factors Affecting TKT and PDC Functioning
TKT and PDC can be inhibited by various factors, including environmental toxins, genetic factors, and lifestyle choices. For example, heavy metals, such as lead and mercury, can impair enzyme function. Genetic mutations can also affect the expression or activity of these enzymes, leading to metabolic disorders. Additionally, factors such as poor diet, alcohol consumption, and chronic stress can negatively impact TKT and PDC functioning.
Thiamine deficiency can lead to numerous health problems, including hypoxia, which is characterized by insufficient oxygen levels in the body’s tissues. In this article, we will explore how thiamine deficiency induces hypoxia and its consequences on overall health.
Thiamine Deficiency and Its Effects on Pyruvate Dehydrogenase Complex (PDC)
Thiamine deficiency can downregulate the activity of the Pyruvate Dehydrogenase Complex (PDC), a crucial enzyme responsible for converting pyruvate into acetyl-CoA, which subsequently enters the Krebs cycle for ATP production. PDC requires thiamine pyrophosphate (TPP), a derivative of thiamine, as a cofactor to function efficiently. Therefore, insufficient thiamine levels can impair PDC activity, leading to a decrease in oxidation and oxygenation processes within the mitochondria.
Environmental and genetic factors that can negatively impact the PDC
Magnesium is required for the activation and phosphorylation of thiamine, which is a critical cofactor for PDC. However, about 50% of the population is deficient in magnesium, which can lead to impaired PDC activity and energy metabolism. Riboflavin and alpha-lipoic acid are also required for the proper functioning of specific subunits of PDC, and their deficiency can impair PDC activity and energy production.
Heavy metals like arsenic and mercury can impair PDC activity by blocking alpha-lipoic acid and inducing hypoxia-inducible factors (HIFs) that impair mitochondrial respiration. These heavy metals can also cause symptoms similar to thiamine deficiency, and increasing thiamine intake can improve these symptoms. Thiamine can also chelate heavy metals, which can help prevent their toxic effects on PDC and energy metabolism.
In summary, the activity of PDC can be affected by various factors, including the availability of essential cofactors like magnesium, thiamine, riboflavin, and alpha-lipoic acid, as well as heavy metals like arsenic and mercury. It is essential to maintain optimal levels of these cofactors and avoid exposure to heavy metals to support proper PDC activity and energy metabolism.
Impaired Oxidation and Oxygenation
Oxidation refers to the mitochondria’s ability to utilize oxygen efficiently to produce ATP through oxidative phosphorylation . When PDC activity is impaired due to thiamine deficiency, the body’s ability to generate ATP is compromised, leading to decreased energy production.
Oxygenation is the process of transporting oxygen throughout the body, and it is an ATP-dependent function. Thiamine deficiency can affect arterial oxygen saturation and venous oxygen levels, resulting in inadequate oxygen supply to the body’s tissues. This can manifest as ‘air hunger’ or the sensation of not getting enough oxygen, which may initially present as a ‘happy hypoxic/hypoxemic’ state, where individuals may not initially show signs of distress despite low oxygen levels.
Thiamine deficiency can have profound effects on the body’s ability to produce energy and transport oxygen efficiently, ultimately leading to hypoxia. Ensuring adequate thiamine intake through diet or supplementation is crucial for maintaining optimal cellular function and preventing the detrimental effects of hypoxia on overall health.
Thiamine deficiency and the standard American diet
Thiamine deficiency is a common issue associated with the standard American diet, which is often heavy in seed oils, high-fructose corn syrup (HFCS), and low in essential nutrients. The intake of these food components can lead to several metabolic dysfunctions, including the upregulation of pyruvate dehydrogenase kinases (PDKs) that inactivate pyruvate dehydrogenase complex (PDC). PDKs are also upregulated in the tumor environment and stabilize hypoxia-inducible factor 1 alpha (HIF1a), which can lead to an increased risk of cancer development.
Seed oils, such as soybean, corn, and canola oils, are commonly used in the standard American diet for cooking and food processing due to their high omega-6 fatty acid content. However, excessive consumption of these oils can cause inflammation and insulin resistance, leading to an increased risk of chronic diseases like cardiovascular disease, obesity, and type 2 diabetes. Additionally, studies have shown that seed oil consumption upregulates PDKs, which inactivate PDC, a critical enzyme involved in the breakdown of glucose for energy production.
HFCS is a common sweetener used in the standard American diet in various processed foods and drinks. It is made from cornstarch, which is chemically modified to increase its fructose content. The consumption of HFCS has been linked to several metabolic dysfunctions, including increased insulin resistance, inflammation, and oxidative stress, which can lead to an increased risk of chronic diseases like obesity and diabetes. HFCS also uses more ATP than it produces, which can lead to a depletion of ATP and impaired energy metabolism in the body.
Furthermore, the standard American diet is generally low in essential nutrients, including thiamine, which is necessary for the metabolism of glucose and the production of energy in the body. A diet low in thiamine can lead to a condition known as beriberi, which is characterized by fatigue, muscle weakness, and nerve damage. The relative lack of essential nutrients in the standard American diet, coupled with its high caloric and toxicant load, can also contribute to malnutrition and an increased risk of chronic diseases.
How common is thiamine deficiency?
In general, Thiamine deficiency is a significant public health concern that can affect various populations, including obese patients, elderly individuals, psychiatric patients, ER patients, ICU patients, pregnant women, and those with congestive heart failure and hyperemesis. However, we believe that there exists a subclinical vitamin deficiency which is common in the general population. Nevertheless, the prevalence of thiamine deficiency varies among these populations, as described below:
-
Obese patients: Thiamine deficiency is prevalent in 15-29% of obese patients pre-bariatric surgery, and the incidence increases to approximately 49% post-surgery.
-
Elderly individuals: Thiamine deficiency is prevalent in 20-50% of community-dwelling elderly individuals and up to 45% of elderly patients in acute care.
-
Psychiatric patients: Thiamine deficiency is prevalent in 30% of psychiatric patients.
-
ER patients: Thiamine deficiency is prevalent in 20% of ER patients based on a random sample.
-
ICU patients: Thiamine deficiency is prevalent in 10% of ICU patients upon admission, which increases to 20% after three days and up to 70% if the patient is septic.
-
Congestive heart failure patients: Thiamine deficiency is prevalent in up to 91% of congestive heart failure patients.
-
Pregnant women: Thiamine deficiency is prevalent in 38% of pregnant women.
-
Hyperemesis: Thiamine deficiency is prevalent in 47-63% of hyperemesis cases, but it is likely more prevalent as it is not often measured until Wernicke’s encephalopathy (WE) is present.
Medications and thiamine deficiency:
Several common medications can deplete thiamine levels in the body, which can lead to thiamine deficiency and its associated health complications. The following medications have been identified as potential thiamine depleters:
-
Metformin: Metformin is a commonly prescribed medication for the treatment of type 2 diabetes. It can interfere with thiamine absorption and increase thiamine excretion in the urine, leading to thiamine deficiency.
-
Psychiatric medications: Several psychiatric medications, including antipsychotics and antidepressants, have been shown to deplete thiamine levels in the body, leading to thiamine deficiency.
-
Antibiotics: Antibiotics like metronidazole and trimethoprim can interfere with thiamine absorption and metabolism, leading to thiamine deficiency.
-
Nonsteroidal anti-inflammatory drugs (NSAIDs), acetaminophen, and aspirin: These medications can interfere with thiamine absorption and metabolism, leading to thiamine deficiency.
-
Proton pump inhibitors (PPIs): PPIs are commonly prescribed medications for the treatment of acid reflux and ulcers. They can interfere with thiamine absorption and metabolism, leading to thiamine deficiency.
-
Diuretics: Diuretics are commonly prescribed medications for the treatment of high blood pressure and edema. They can interfere with thiamine absorption and metabolism, leading to thiamine deficiency.
-
Chemotherapeutic drugs: Chemotherapeutic drugs like 5-fluorouracil can interfere with thiamine absorption and metabolism, leading to thiamine deficiency.
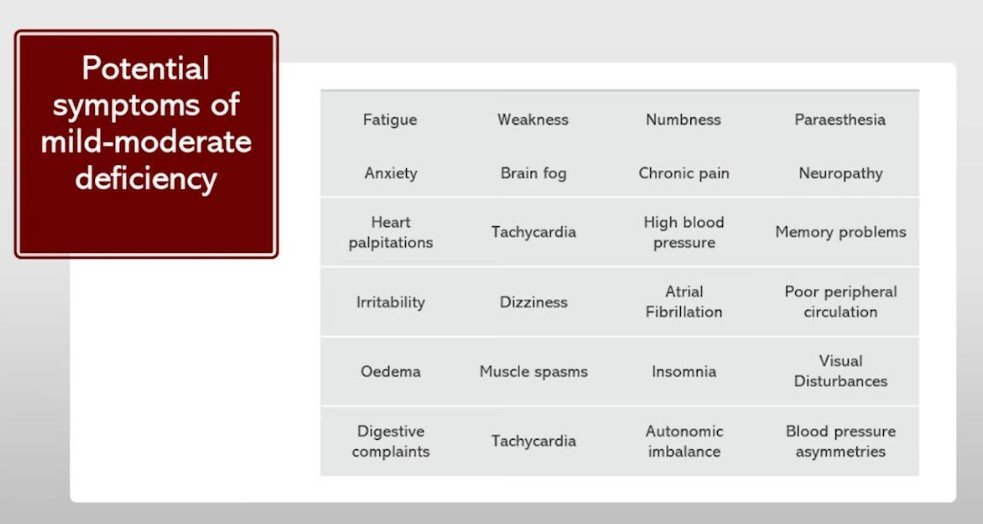
What are some of the symptoms of thiamine deficiency?
Conventional medicine only recognizes acute situations of thiamine deficiency such as beriberi which is exceedingly rare in modern times. However, the possibility exists for a low-grade subclinical thiamine deficiency affecting multiple body systems. Most of what we observe in the clinical setting are mild to moderate symptoms which can easily be missed by the standard medical model.

Image taken from Vitamin B1 (Thiamine) Deficiency: Cardiovascular & Circulatory Diseases, EON Nutrition, https://www.youtube.com/watch?v=gCoxSsA5OG0
The typical most presenting symptoms are subclinical neurological symptoms– typically fatigue, dizziness, etc. In any regard, thiamine deficiency can lead to a wide range of symptoms, ranging from mild to severe.
The following symptoms are commonly associated with thiamine deficiency:
-
Fatigue, exercise intolerance, and decreased wakefulness: Thiamine deficiency can impair energy metabolism, leading to fatigue and exercise intolerance. It can also affect the function of orexin/hypocretin neurons in the brain, which regulate wakefulness and can lead to decreased wakefulness.
-
Cyclic vomiting, gastroparesis, and pseudo bowel obstructions: Thiamine deficiency can impair the function of the digestive system, leading to cyclic vomiting, gastroparesis, and pseudo bowel obstructions.
-
Myalgia, myopathy, and neuropathy: Thiamine deficiency can cause muscle pain, weakness, and nerve damage, leading to myalgia, myopathy, and neuropathy.
-
Migraines, seizures, and syncope: Thiamine deficiency can affect the function of the nervous system, leading to migraines, seizures, and syncope.
-
Ataxia and tremors: Thiamine deficiency can impair the function of the cerebellum, leading to ataxia and tremors.
-
Hormone disorders: Thiamine deficiency can affect hormone regulation, leading to hormone disorders such as adrenal insufficiency and hypothyroidism.
-
Depression, mania, anxiety, and psychosis: Thiamine deficiency can affect mental health, leading to depression, mania, anxiety, and psychosis.
-
Cognitive impairment: Thiamine deficiency can impair cognitive function, leading to memory loss, confusion, and other cognitive impairments.
-
Diabetes, metabolic, inflammatory, and immune dysfunction: Thiamine deficiency can impair insulin sensitivity, leading to an increased risk of diabetes. It can also affect metabolism, inflammation, and immune function, leading to various health complications.
Other symptoms of thiamine deficiency include irritability, personality changes, weight loss, and respiratory distress. In severe cases, thiamine deficiency can lead to life-threatening conditions such as Wernicke’s encephalopathy and Korsakoff’s syndrome.
Thiamine Supplementation and Its Benefits
Thiamine supplementation can offer various potential benefits for individuals with thiamine deficiency and other health conditions, including:
-
Improved energy metabolism: Thiamine is essential for energy production, and supplementation can improve energy metabolism in the body.
-
Improved neurological function: Thiamine is crucial for nerve function, and supplementation can improve cognitive function and reduce the risk of neurological disorders.
-
Improved cardiovascular health: Thiamine supplementation can improve cardiovascular function and reduce the risk of heart disease.
-
Improved glucose metabolism: Thiamine supplementation can improve glucose metabolism and reduce the risk of diabetes and its associated complications.
-
Improved immune function: Thiamine is essential for immune function, and supplementation can improve immune function and reduce the risk of infections.
-
Improved mood: Thiamine is crucial for mental health, and supplementation can improve mood and reduce the risk of depression and anxiety.
-
Improved digestion: Thiamine is necessary for digestive function, and supplementation can improve digestion and reduce the risk of digestive disorders.
Not all thiamine supplements are created the same
Ordinary thiamine supplements, which often come in the form of thiamine salts, may not be the most effective way to increase thiamine levels in the body and the brain. This is because the absorption and bioavailability of these supplements are limited by a rate-limiting transport system.
Benfotiamine is a fat-soluble form of thiamine that is more easily absorbed by the body than thiamine HCl, which is the more common water-soluble form of thiamine. Benfotiamine is a synthetic derivative of thiamine that is created by attaching a lipid group to the thiamine molecule. This lipid group allows benfotiamine to pass through cell membranes more easily and be absorbed more efficiently by the body.
Due to its increased absorption and bioavailability, benfotiamine is often used as a form of thiamine supplementation to treat various health conditions, including diabetic neuropathy, sciatica, and chronic pain. Studies have also shown that benfotiamine can improve glucose metabolism and nerve function, making it an effective treatment for diabetes-related complications.
Thiamine tetrahydrofurfuryl disulfide (TTFD), on the other hand, is a powerful thiamine derivative that bypasses these limitations. TTFD increases thiamine levels in the body more effectively than traditional thiamine salts. It also possesses antioxidant and anti-inflammatory properties that can contribute to overall health.
One of the most significant benefits of TTFD is its ability to cross the blood-brain barrier, rapidly saturating brain cells with thiamine. This quality makes TTFD a superior alternative to other forms of thiamine for restoring and maintaining healthy thiamine levels in the brain and central nervous system.
Unfortunately, there are various forms of TTFD that contain various fillers and flow agents, such as magnesium stearate, that are not ideal for long-term supplementation. However, there is a brand of TTFD without these called Thiamax, containing 100 mg of pure TTFD.
Dosing
NOTE: This information is not intended for medical use or to assist with self-treatment but only for educational purposes only. Always seek the care of a medical provider when doing anything in support of your own health.
First of all, we always recommend working with a medical professional to determine if a thiamine deficiency is present and being extraordinarily careful with starting therapy with thiamine, as detox and healing reactions can occur with higher dosages. In many cases, one may feel worse before they start to feel better on this therapy, and careful monitoring is always recommended. In any regard, the recommended starting dose of TDFD is 50 to 100 mg, slowly building to 200 to 500 mg per day. If you are dealing with any type of insulin resistance, prediabetes or diabetes do consider supplementing also with Benfotiamine.
Please note that it is very important to have all the cofactors supplemented– this includes most of the B vitamins to support the Krebs cycle, adequate magnesium, in particular magnesium malate, as well as supplemental magnesium and potassium. We always recommend monitoring all micronutrients during this process as well as taking a daily journal of symptom improvements.
Initial symptom improvements can include improvements in energy and fatigue, brain fog, balance, and gait improvements, improvements in peripheral edema, and improvements in symptoms of POTS, tachycardia, blood pressure, etc.
Conclusion
Thiamine is an essential nutrient that plays a pivotal role in maintaining our health and well-being. By supporting a myriad of metabolic processes and maintaining the proper functioning of our nervous system, thiamine ensures that our bodies operate at peak efficiency. Thiamine supplementation, particularly in the form of TTFD, can help improve overall health and protect against the consequences of thiamine deficiency. By unlocking the power of thiamine supplementation, you can support your body’s metabolic processes, maintain a healthy nervous system, and enjoy the numerous benefits of this essential nutrient. If you are wondering if you have a thiamine deficiency and or may be looking for high-dose thiamine support, please do not hesitate to contact our office for further evaluation and treatment.
To learn more
-
The Most UNDERRATED and OVERLOOKED B Vitamin Deficiency is Thiamine https://www.youtube.com/watch?v=FsXNw2cAw5c&t=10s
-
Better Health Guy Episode #163: Thiamine Deficiency Disease with Dr. Chandler Marrs, MS, MA, PhD https://www.youtube.com/watch?v=b1SSKBZp8D8&t=3s
-
Chandler Marrs, PhD https://www.hormonesmatter.com/author/chandler/
-
Must Read: Thiamine Deficiency Disease, Dysautonomia, and High Calorie Malnutrition
by Derrick Lonsdale and Chandler Marrs | Jul 18, 2017
-
Elliot at EON Nutrition: https://www.youtube.com/@EONutrition
References:
Bettendorff, L., & Wins, P. (2009). Thiamin diphosphate in biological chemistry: new aspects of thiamin metabolism, especially triphosphate derivatives acting other than as cofactors. FEBS Journal, 276(11), 2917-2925.
Bivona L, Persaud Y. Thiamine (Vitamin B1). StatPearls Publishing; 2021.
Brown, G. C., & Cooper, C. E. (1994). Nanomolar concentrations of nitric oxide reversibly inhibit synaptosomal respiration by competing with oxygen at cytochrome oxidase. FEBS Letters, 356(2-3), 295-298.
Butterworth RF. Thiamin deficiency and brain disorders. Nutr Res Rev. 2003;16(2):277-284.
Costantini, A., Pala, M. I., Tundo, S., & Matteucci, P. (2013). High-dose thiamine improves the symptoms of irritable bowel syndrome. BMJ Case Reports, 2013, bcr2013009280.
Costantini, A., Romani, A., Mencacci, N. E., & Del Dotto, P. (2016). Dystonia and thiamine: A new therapeutic approach. Movement Disorders Clinical Practice, 3(6), 614-616.
Dabar, George & Harmouche, Carine & Habr, Bassem & Riachy, Moussa & Jaber, Bertrand. (2015). Shoshin Beriberi in Critically-Ill patients: Case series. Nutrition journal. 14. 51. 10.1186/s12937-015-0039-7.
Díaz-Muñoz, M., & Moreno-Sánchez, R. (1990). Kinetic studies of the transketolase in extracts of maize leaves. Plant physiology, 93(2), 747-754.
DiNicolantonio JJ, O’Keefe JH. Omega-6 vegetable oils as a driver of coronary heart disease: the oxidized linoleic acid hypothesis. Open Heart. 2018;5(2):e000898.
Frank, R. A., Leeper, F. J., & Luisi, B. F. (2007). Structure, mechanism and catalytic duality of thiamine-dependent enzymes. Cellular and Molecular Life Sciences, 64(7-8), 892-905.
Harper, A. E., Miller, R. H., & Block, K. P. (1984). Branched-chain amino acid metabolism. Annual Review of Nutrition, 4(1), 409-454.
http://thiamine.dnr.cornell.edu/Thiamine_biochemistry.html
Kish SJ. Biochemistry of thiamine: Metabolism, deficiency, and disease. In: Annals of the New York Academy of Sciences. Vol. 585. New York Academy of Sciences; 1990:1-16.
Kumar PA, Kumar A, Kumaravel TS. Heavy metals, their toxic effects on living cells and their detoxification: a review. In: First International Conference on Interdisciplinary Research and Development. Vol 1. IOP Publishing; 2020:012002.
Lee J, Cho Y, Kim J, Park J. Thiamine deficiency and cancer: a review. Cancer Res Treat. 2019;51(3):677-686.
Lee, J. Y., Kim, Y. R., Kang, H. M., Kim, H. J., Park, B. J., & Kim, Y. (2019). Thiamine levels are associated with cognitive function in patients with multiple sclerosis. Journal of clinical neurology, 15(3), 328-334.
Lonsdale, D. (2006). A review of the biochemistry, metabolism and clinical benefits of thiamin(e) and its derivatives. Evidence-Based Complementary and Alternative Medicine, 3(1), 49-59.
Lonsdale, D. (2006). A review of the biochemistry, metabolism and clinical benefits of thiamin (e) and its derivatives. Evidence-Based Complementary and Alternative Medicine, 3(1), 49-59.
Lonsdale, D. (2017). Thiamine Deficiency Disease, Dysautonomia, and High Calorie Malnutrition. Elsevier.
Martin, D. S., Grocott, M. P., & Levett, D. Z. (2010). Oxygen therapy and anaesthesia: too much of a good thing? Anaesthesia, 65(4), 341-343.
Mitchell, P. (1961). Coupling of phosphorylation to electron and hydrogen transfer by a chemi-os
Patel, M. S., & Korotchkina, L. G. (2006). Regulation of the pyruvate dehydrogenase complex. Biochemical Society Transactions, 34(Pt 2), 217-222.
Sazanov, L. A. (2015). A giant molecular proton pump: structure and mechanism of respiratory complex I. Nature Reviews Molecular Cell Biology, 16(6), 375-388.
Sechi, G., & Serra, A. (2007). Wernicke’s encephalopathy: new clinical settings and recent advances in diagnosis and management. The Lancet Neurology, 6(5), 442-455.
Stincone, A., Prigione, A., Cramer, T., Wamelink, M. M., Campbell, K., Cheung, E., … & Selivanov, V. (2015). The return of metabolism: biochemistry and physiology of the pentose phosphate pathway.
Tobin, M. J., & Jubran, A. (2020). Counter



